FIG. 1 shows a transmission electron microscopic (TEM) photo of the styrene oligomer composite with nano Pd particles deposited on the surface from the nanofabrication process developed at Chung Cheng Institute of Technology.
Chung Cheng Institute of Technology, National Defense University (Taoyuan, TW) in U.S. Patent , chemists divulge a simple process for preparing a metal styrene polymer composite having nano metallic particles deposited on them.
Inventors Jinn-Luh Ou, Chang-Ping Chang, Yuh Sung, Ming-Der Ger, Chun-Chieh Tseng and Wen-Ding Chen developed the process for preparing a metal styrene polymer composite in which nano metallic particles are deposited.
The nanofabrication steps include: a) undergoing free radical polymerization of styrene and an optional co-monomer in the presence of a persulfate initiator and a chain transfer agent; and b) contacting the resulting styrene oligomer or copolymer of styrene and the co-monomer from step a) with an aqueous solution containing a noble metal ion dissolved therein, so that the noble metal ion is reduced to element form particles and deposit on the styrene oligomer or copolymer of styrene and the co-monomer by sulfates on the oligomer or copolymer in the absence of a reducing agent.
More specifically, a process for preparing a metal styrene polymer composite with metallic nano particles deposited on the surface. The styrene polymer can be a styrene oligomer or a copolymer of styrene and a hydrophilic monomer. Furthermore, the styrene oligomer can be further grafted with hydrophilic groups in order to improve the hydrophilic property of the metal styrene oligomer composite with metallic nano particles deposited on the surface thereof.
The process according comprises polymerizing styrene monomer and, optionally, a hydrophilic monomer in the presence of a persulfate initiator and a chain transfer agent by free radical polymerization; contacting the resulting styrene oligomer or copolymer with a solution containing metallic ions at an elevated temperature, thereby forming by reduction metallic nano particles on the surface of the styrene oligomer or copolymer. The persulfate initiator used in the process of the present invention has reducing power under high temperature. As a result, metal ions can be reduced into elemental metal without the use of a reducing agent. Furthermore, the use of the chain transfer agent enables the styrene monomer to form an oligomer with a low molecular weight during the free radical polymerization, thereby rendering a reduced hydrophobic property of the resulting composite.
Optionally, the invented process further comprises modifying said composite to improve the hydrophilic property. For example, a sulfonate group is grafted onto the para-position of the styrene oligomer.
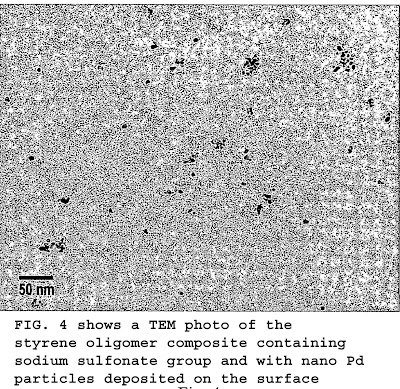
FIG. 4 shows a TEM photo of the styrene oligomer composite containing sodium sulfonate group and with nano Pd particles deposited on the surface
Optionally, the invented process further comprises modifying said composite to improve the hydrophilic property. For example, a sulfonate group is grafted onto the para-position of the styrene oligomer.
FIG. 4 shows a TEM photo of the styrene oligomer composite containing sodium sulfonate group and with nano Pd particles deposited on the surface
Nano composites involve many aspects of technology and have a wide variety of production processes available. In brief, the processes for producing nano composite can be classified into four groups: directly mixing metallic nano particles with polymer(s); forming metallic nano particles in a polymer matrix; polymerizing a polymer from monomer(s) in the presence of metallic nano particles; simultaneously forming metallic nano particles and a polymer.
In the processes for preparing nano composite, the most crucial issue involves an effective control on the dimensions of the metallic nano particles per se in order to ensure that at least one dimension of a composition phase in the composite is within the range of a nano dimension, and then care is taken to control the aggregation of nano particles.
In general, a surfactant, a metal salt, and a reducing agent are used in a chemical reduction process for producing nano particles. The types of applicable reducing agents include: hydrazine, sodium borohydride, hydrogen, etc. A typical chemical reduction process for producing nano particles comprises dissolving a surfactant in a liquid phase; adding and mixing metal ions in the liquid phase; and adding a reducing agent into the resulting mixture to reduce the metal ions into nano particles.